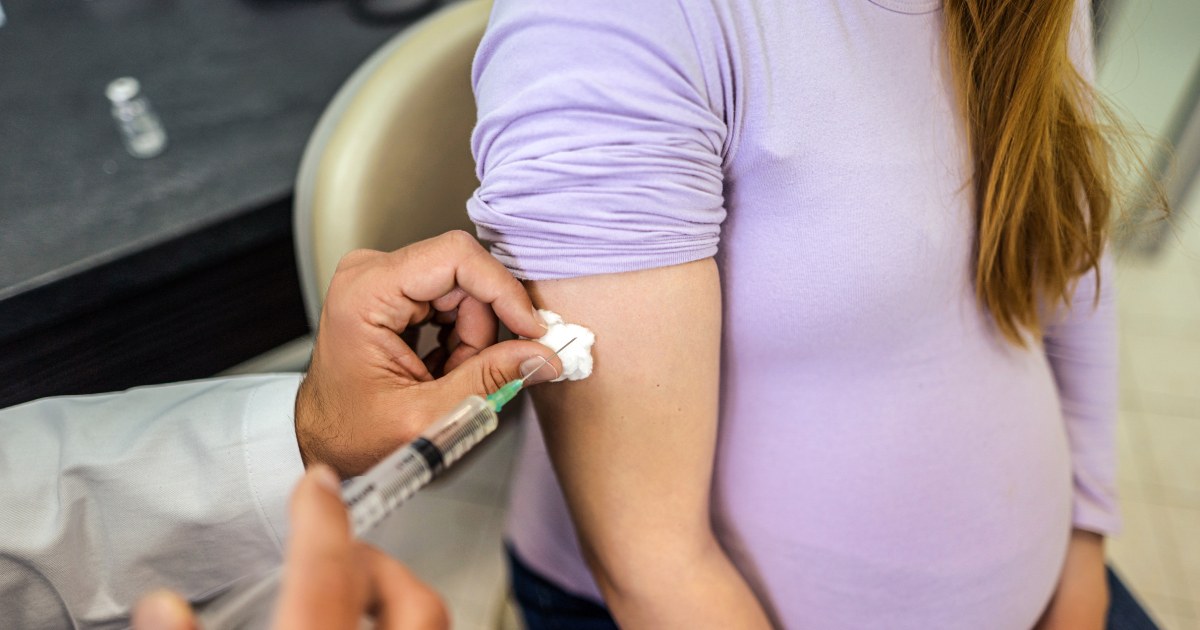
Pregnant people should get an RSV vaccine at 32 to 36 weeks’ gestation in order to protect their newborns from RSV, the Centers for Disease Control and Prevention said Friday.
An advisory committee to the agency voted 11-1 to recommend the injection. Soon after, CDC Director Mandy Cohen formally endorsed that recommendation, the final step that allows the vaccine to be distributed to the public.
The single-dose shot, made by Pfizer, is called Abrysvo and already approved by the Food and Drug Administration. It spurs the production of antibodies in the mother that transfer through the placenta. The vaccine is the first approved that can protect babies from RSV.
That protection lasts through their first six months, clinical trial data showed. When given at 32 to 36 weeks’ gestation, Abrysvo was found to lower the risk of severe disease from RSV among infants by 91% within roughly three months after birth. By around six months, the efficacy was just under 77%.
RSV is typically mild in young, healthy adults, but it poses a bigger threat to children younger than 5. The virus causes up to 80,000 hospitalizations and up to 300 deaths annually among that age group in the United States. Almost 80% of children hospitalized with RSV under age 2 have no underlying medical conditions.
Last year, a dramatic spike in severe RSV infections overwhelmed children’s hospitals.
RSV is also common — most children are infected in the first year of life, and almost all by age 2.
Pregnant people should get the new vaccine from September through January, when RSV rates are highest nationally, the CDC said.
Cases of RSV are already rising, with the number of positive tests higher now than in mid-August, according to CDC data.
Another new form of RSV protection for babies was also approved this summer: an injectable drug that delivers a dose of antibodies directly to an infant’s bloodstream.
The CDC recommended that shot, called Beyfortus, for all babies up to 8 months old who are born during or entering their first RSV season, as well as for infants 8 to 19 months old who are at increased risk of severe disease and entering their second season.
The CDC advisory committee said Friday that either shot can be used to protect infants, and most babies won’t need both.
The benefits of the vaccine are that it protects babies immediately after birth and doesn’t require them to get a shot. However, the antibody injection doesn’t pose a risk of adverse pregnancy outcomes, and some evidence suggests its protection wanes more slowly.
The CDC advisers also noted that the availability of Beyfortus may be limited during the current RSV season.
Dr. Helen Keipp Talbot, the lone CDC advisory group member who voted against Abrysvo, expressed concern that the recommendation was too complicated.
“I think it’s going to make providing care more difficult,” said Talbot, an associate professor of medicine at Vanderbilt University in Nashville, Tennessee. “If we just say the child should get the monoclonal [antibody drug], we have a very simple recommendation. It’s a cost effective and very effective mechanism of protecting the child.”
But other members said it was important to have more than one protective tool.
“I could not have imagined better options for our infants and children. This is what we’ve been striving for. As a neonatologist with high-risk babies, this is just fantastic,” said Dr. Pablo Sánchez, a pediatrics professor at The Ohio State University College of Medicine.
The most common side effects reported among pregnant women in Pfizer’s trial were fatigue, headache, injection site pain, muscle pain, nausea, joint pain and diarrhea.
There was also a slightly higher rate of preterm births (defined as before 37 weeks’ gestation) among women who got the vaccine than those who got a placebo. The difference wasn’t statistically significant, but the prescribing label for Abrysvo comes with a warning not to administer it before 32 weeks’ gestation nonetheless.
Last year, the pharmaceutical company GSK halted its trial of an RSV vaccine after it showed a higher preterm birth rate among some recipients. The CDC advisers said Friday that those results were relevant to the evaluation of Pfizer’s shot.
Pfizer’s trial also found a slightly higher number of hypertensive disorders like pre-eclampsia among the vaccine group. Pfizer has said it will monitor the risk of preterm birth and hypertensive disorders among vaccine recipients.
Due to a lack of data, the CDC committee said it’s too soon to know whether additional RSV vaccine doses should be given in subsequent pregnancies.
Abrysvo is expected to be listed at $295, but the price for individual consumers will depend on their insurance plans. The shot will be covered under the CDC’s Vaccines for Children Program, which makes some vaccines and immunizations free to children who are uninsured, underinsured, Medicare-eligible, or Native American or Alaska Natives.
Meanwhile, two new RSV vaccines are already available at pharmacies for adults ages 60 and older — a group also vulnerable to severe illness. One of those shots, from Pfizer, is the same formulation as the new shot for use during pregnancy.







Recent Comments