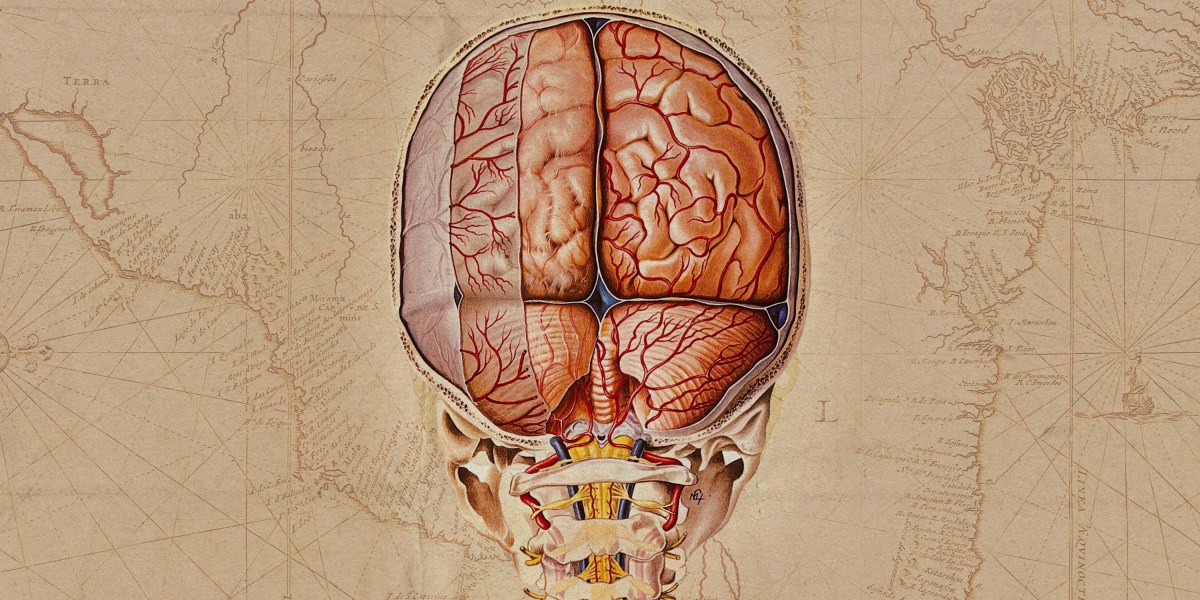
And the location of that complexity is surprising. Neuroscience has focused much of its research on the outer shell of the brain, which is responsible for memory, learning, language, and more. But the majority of cellular diversity is actually in older evolutionary structures deep inside the brain, Lein says.
How did they make these atlases?
The classic neuroscience approach to classifying cell types relies on either cell shape–think of star-shaped astrocytes–or the cells’ type of activity–such as fast-spiking interneurons. “These cell atlases capitalize on a new suite of technologies that come from genomics,” Lein says, primarily a technique known as single-cell sequencing.
First, the researchers start with a small piece of frozen brain tissue from a biobank. “You take a tissue, you grind it up, you profile lots of cells to try to make sense of it,” Lein says. They make sense of it by sequencing the cells’ nuclei to look at the genes that are being expressed. “Each cell type has a coherent set of genes that they typically use. And you can measure all these genes and then cluster all the types of cells on the basis of their overall gene expression pattern,” Lein says. Then, using imaging data from the donor brain, they can put this functional information where it belongs spatially.
How can scientists use these brain cell atlases?
So many ways. But one crucial use is to help understand the basis of brain diseases. A reference human brain atlas that describes a normal or neurotypical brain could help researchers understand depression or schizophrenia or many other kinds of diseases, Lein says. Take Alzheimer’s as an example. You could apply these same methods to characterize the brains of people with differing levels of severity of Alzheimer’s, and then compare those brain maps with the reference atlas. “And now you can start to ask questions like, ‘Are certain kinds of cells vulnerable in disease, or are certain kinds of cells causal,” Lein says. (He’s part of a team that’s already working on this.) Rather than investigating plaques and tangles, researchers can ask questions about “very specific kinds of neurons that are the real circuit elements that are likely to be perturbed and have functional consequences,” he says.
What’s the next step?
Better resolution. “The next phase is really moving into very comprehensive coverage of the human and non-human primate brain in adults and development.” In fact, that work has already begun with the BRAIN Initiative Cell Atlas Network, a five-year, $500 million project. The aim is to generate a complete reference atlas of cell types in the human brain across the lifespan, and also to map cell interactions that underlie a wide range of brain disorders.





Recent Comments