The Food and Drug Administration could expand the use of Pfizer-BioNTech’s Covid-19 vaccine to children ages 6 months to 5 years by the end of February, a person with knowledge of the plan said.
Federal regulators want to begin reviewing the data on two doses of the vaccine for this age group while the companies continue to gather data on a potential three-dose regimen, the person said.
Full coverage of the coronavirus outbreak
Pfizer said in December it was testing a third dose of its vaccine in an ongoing trial of young children after it found that the two-dose regimen didn’t generate a strong enough immune response in some children.
The FDA is expected to eventually sign off on three doses for kids under 5, but regulators believe two doses in the meantime should provide enough — though less than ideal — protection against the omicron variant, the individual said.
The news was first reported by The Washington Post.
The FDA’s plan may come as a relief to parents wanting to get their young children vaccinated.
Omicron accounts for virtually all new Covid cases in the United States, according to the Centers for Disease Control and Prevention. The new variant has led to a dramatic spike in pediatric cases.
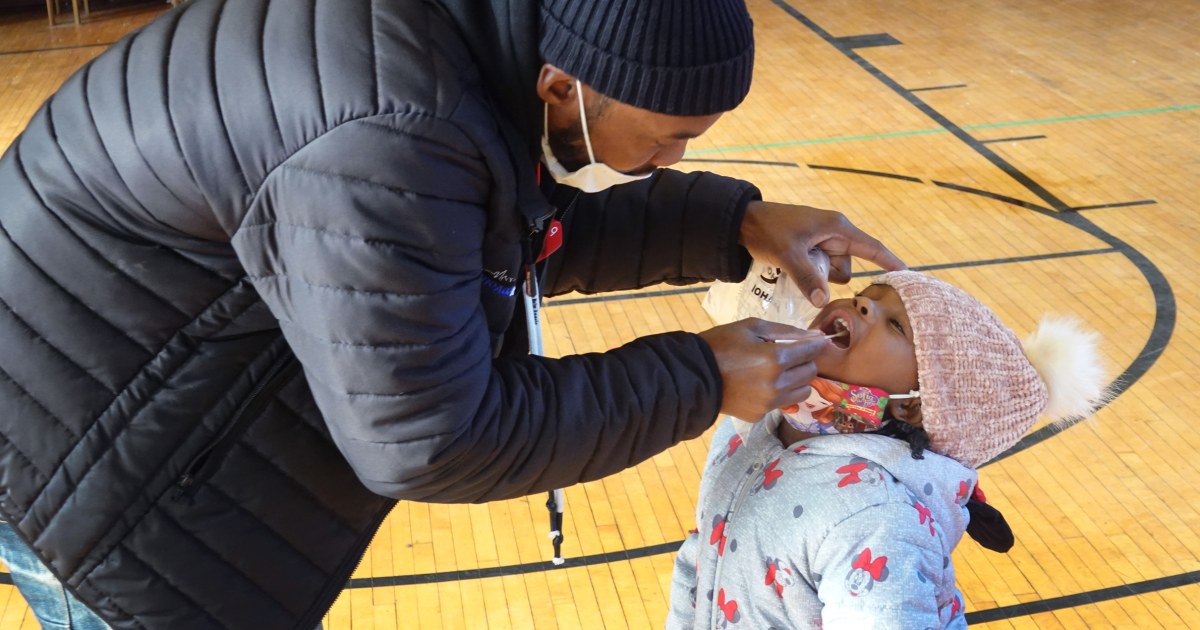
Children under 5 are the only group in the U.S. not authorized to receive a Covid vaccine. Pfizer’s vaccine has been cleared for kids as young as 5, while Moderna and Johnson & Johnson’s vaccines are authorized for adults.
Pfizer has not yet filed a submission to the FDA to authorize its vaccine for kids under 5, the company said in a statement Monday. “We’re continuing to collect and analyze data from both two and three doses in our younger age cohort,” the company said.
However, two people familiar with the plans say the submission is expected this week, possibly as early as Tuesday.
There are an estimated 19.5 million children under 5 in the U.S. and Puerto Rico, according to NBC News data.
Pfizer has been testing 3 microgram doses for the age group. Kids 5 to 11 have been authorized to receive 10 microgram doses, while everyone ages 12 and up receives 30 microgram doses.
Follow NBC HEALTH on Twitter & Facebook.
Heidi Przybyla contributed.






Recent Comments