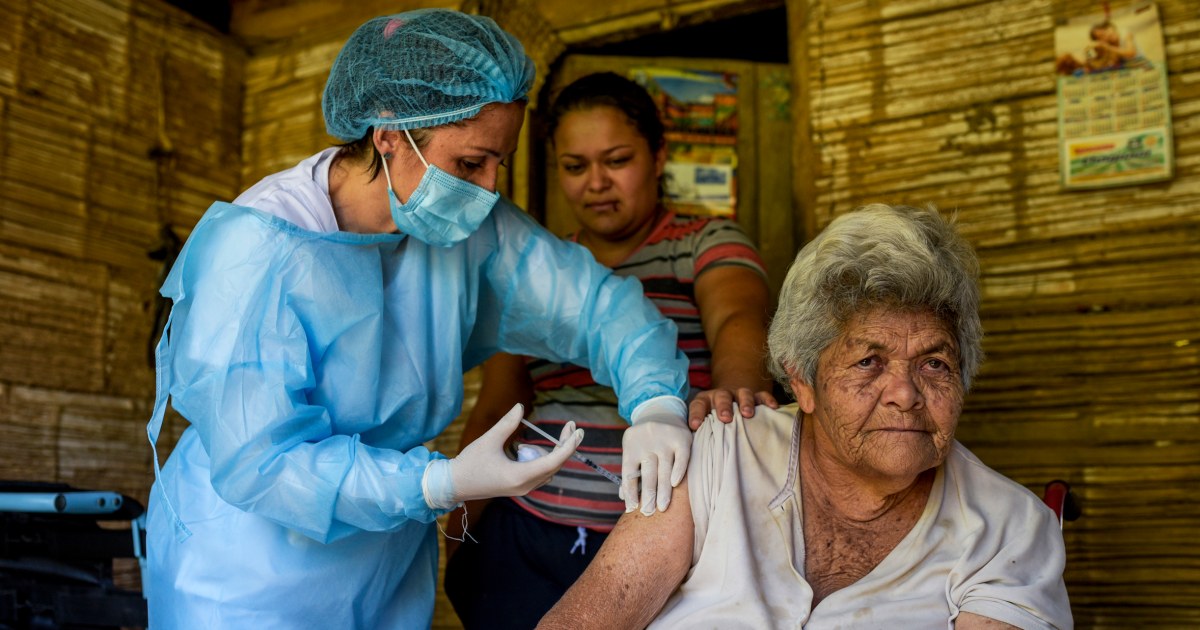
Novavax asked the Food and Drug Administration on Monday to authorize its protein-based Covid-19 vaccine for adults.
The vaccine is already available for use in at least 170 countries, but if cleared for emergency use in the United States, it would provide an alternative to the popular mRNA-based shots from Pfizer-BioNTech and Moderna.
Full coverage of the coronavirus outbreak
For certain groups of people — particularly young men — the mRNA vaccines carry a slightly elevated risk of a heart condition called myocarditis. Novavax’s vaccine has not been linked to myocarditis.
The decision will now go to the FDA, which is expected to take several weeks to review the application. Novavax CEO Stanley Erck has said the vaccine could be authorized by U.S. regulators as early as February.
Clinical trial results, published in the New England Journal of Medicine in December, found two doses of Novavax’s vaccine, given 21 days apart, were safe and highly effective against moderate to severe disease.
The Gaithersburg, Maryland-based biotech company has also said two doses of its vaccine triggered a strong immune response against the Covid omicron variant, though three doses appeared to be better. Still, Novavax said it is developing a modified version of its vaccine tailored to protect against the extremely contagious strain.
Unlike the mRNA vaccines, which trick the body into producing a harmless piece of the virus, triggering an immune response, the Novavax vaccine introduces synthesized coronavirus proteins to kick-start the body’s immune system. It uses an older technology found in other widely used vaccines, like the flu shot.
The biotech company’s FDA request comes as more than 211 million Americans are already fully vaccinated, mainly with doses of the Pfizer-BioNTech and the Moderna vaccines. But the Novavax vaccine offers another alternative to those who are unable or unwilling to get the mRNA shots, experts say.
Some members of the public “may be more accepting of this type of vaccine because it doesn’t have components of messenger RNA,” said Dr. Chris Ohl, an infectious disease expert at Wake Forest Baptist. “It’s a hypothesis that should be tested, but it’s really going to require the company and public health officials to make sure people understand the differences between the vaccines.”
Dr. Paul Offit, a vaccine expert at Children’s Hospital of Philadelphia, said a wider selection of Covid vaccines is important, especially as the mRNA shots have been linked to a rare risk of myocarditis, and Johnson & Johnson’s vaccine has been associated with the risk of rare blood clots.
“I think the more the merrier,” he said. “The more vaccines that you have, you may find differences for certain age groups. There may be different efficacy for high-risk conditions like people who are immunocompromised. There may be different safety profiles.”
The U.S. government and Novavax reached a deal in 2020 for 110 million doses of the vaccine. Since then, the company has suffered from repeated delays in ramping up production of its vaccine and seeking regulatory authorization.
Novavax said Monday a booster study is ongoing to evaluate the safety and effectiveness of a third dose of the vaccine, as well as a study in adolescents ages 12 to 17.
Follow NBC HEALTH on Twitter & Facebook.







Recent Comments