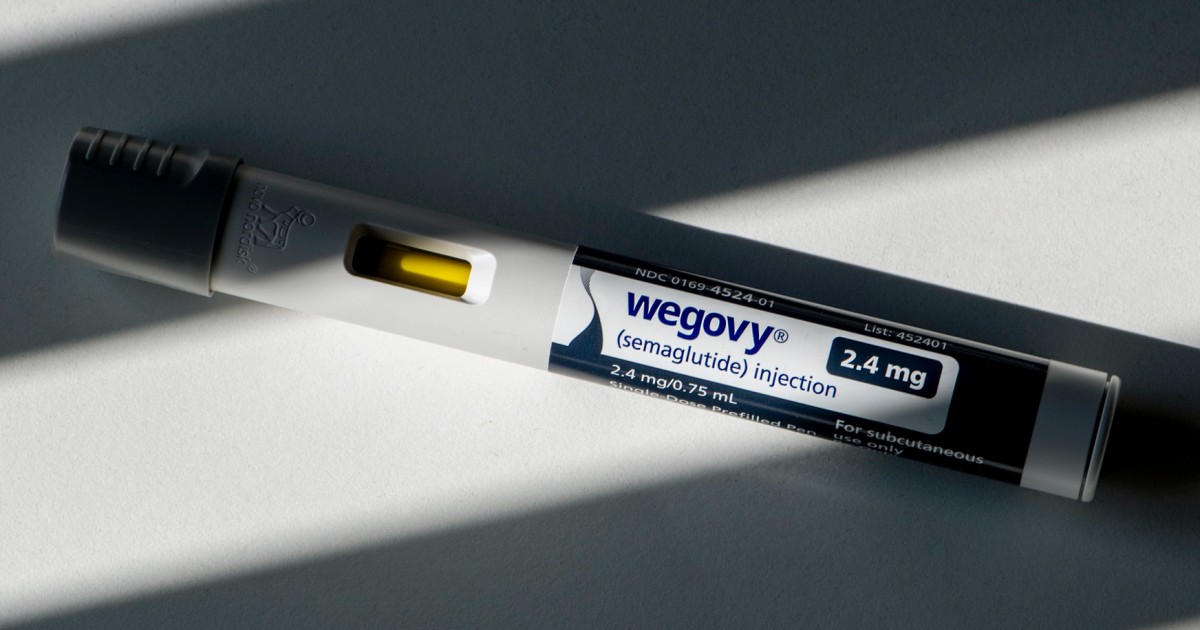
The blockbuster weight loss drug Wegovy is now approved to reduce heart disease risk, drugmaker Novo Nordisk said Friday.
The Food and Drug Administration has updated the drug’s label, saying that Wegovy can be prescribed to reduce a person’s risk of heart attack and stroke, the company said.
Heart disease is the leading cause of death in the United States, according to the Centers for Disease Control and Prevention.
The change comes after, in a late-stage clinical trial of more than 17,000 adults, Novo Nordisk found that Wegovy cut the risk of cardiovascular events — such as heart attack and stroke — by 20% compared to a placebo. All of the people in the trial were overweight or had obesity, and had a history of heart disease.
The label change could mean that more employers and insurers are persuaded to cover the medication.
Many have been hesitant to cover the pricy drug for weight loss.
Wegovy, along with other weight loss medications, such as Eli Lilly’s Zepbound, are typically not covered by insurance; Medicare, by law, is barred from covering them.
The approval, Novo Nordisk said, makes Wegovy the first therapy approved in the United States to help people manage their weight and reduce heart disease risk.
Wegovy contains semaglutide, the same active ingredient found in the popular diabetes drug Ozempic, which is often prescribed off-label for weight loss. Both are manufactured by Novo Nordisk.







Recent Comments